Benzoic Acid Organic or Inorganic
Algae essential oils inorganic salts and oxides derived from minerals natural emollients dyes emulsifiers eg. Ionic Hydrogen bond Dipole van der Waals forces.
Illustrated Glossary Of Organic Chemistry Benzoic Acid Benzoate Benzoate Group
Sulfuric Acid is a mineral acid with the chemical formula H 2 SO 4.
. As examples ethanoic acid is soluble in water. Organic bases can be solubilized in a similar way except that now the solution must be made acidic. Concentrated nitric acid 68 - 70 is a transparent colorless or yellowish fuming suffocating hygroscopic corrosive liquidThis chemical attacks almost all metals.
The section contains Organic Chemistry questions and answers on preparation and reactions of alcohols phenols ethers oxiranes glycols glycerol aldehydes ketones benzaldehyde. Salts of benzoic acid are. 23 Pergamon Press Oxford UK 1979.
The Journal of Organic Chemistry Articles ASAP Perspective Publication Date Web. Rated in order from strongest to weakest these forces are. In contrast to strong inorganic acids such as HCl or HNO 3.
You must have seen an equation like this in one of the introductory biology classes. Sulfuric acid H 2 SO 4 is a strong acid with hygroscopic and oxidizing properties. Buy Nitric Acid Products Online Here Or By Phone.
6CO 2 6H 2 O energy C 6 H 12 O 6 6O 2. At higher concentrations it acts as an oxidizing agent and dehydrating agent. Hyaluronic acid among others is obtained this way.
Ketone and AcidEster-Substituted Free NH Indoles with Iodoarenes via a Palladium Catalyst System. Functional groups are also indicators. In addition to raw materials of plant and animal origin in natural cosmetics you can also find.
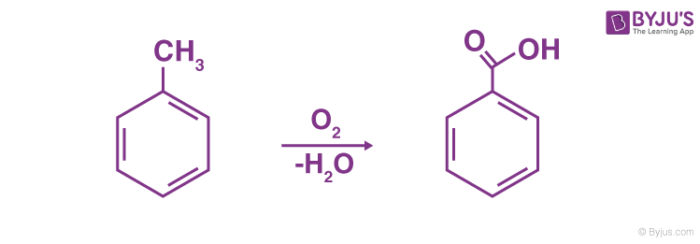
Ester and anhydride compounds Aldehyde and ketone compounds Amine compounds Basic organic raw materials Heterocyclic compounds Hot Products Oxalic Acid NEW. ɪ k is a white or colorless solid with the formula C 6 H 5 CO 2 H. Aldehydes tautomerism physical and chemical properties of carboxylic acids aldehydes ketones acid.
Dempsey eds Ionization Constants of Organic Acids in Solution IUPAC Chemical Data Series No. Organic Chemistry Multiple Choice Questions on Oxygen Containing Organic Compounds. A of its conjugate acid as pK b 14 - pK a.
If you have a mixture. Yunus Taskesenligil Murat Aslan Tuba Cogurcu and. The strength of a base is related to the pK a of its conjugate acid as pK b 14 - pK a.
You can determine which molecule has the higher boiling point by knowing which bonds require more energy in order for the gas phase to be achieved. It is the simplest aromatic carboxylic acidThe name is derived from gum benzoin which was for a long time its only sourceBenzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites. The present review highlights the recent applications of biochar in removing organic and inorganic pollutants present in industrial effluents.
Different kind of sea components eg. The organic acid will separate from solution again. An organic acid anhydride citation needed is an acid anhydride that is an organic compoundAn acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom.
Alcohols carboxylic acids carboxylic acid chlorides amines esters are usually soluble in water. Both of compounds are carboxylic acids. Sulfuric acid is also known as Mattling acid or Oil of vitriol.
Nurullah Saracoglu The Journal of Organic Chemistry Articles ASAP Article. She finished her PhD research with Prof. But when those compounds molecular mass increases solubility in water is decreased.
Erin is a synthetic chemist focused on uniting areas of synthetic organic chemistry photochemistry inorganic materials and polymer chemistry for new applications in materials science and synthesis. Tomislav Rovis leveraging photoredox catalysis to develop new synthetic methods for CO bond. Who showed that hydrophobic interaction is the main mechanism involved in the adsorption of ionizable organic pollutants such as benzoic acid o-chlorobenzene acid and p-chlorobenzene acid.
But benzoic acid is not soluble in water. Benzoic acid b ɛ n ˈ z oʊ. For strengths of organic acids see E.
A common type of organic acid anhydride is a carboxylic anhydride where the parent acid is a carboxylic acid the formula of the anhydride being RCO 2 O. Abigail Doyle and Prof. Inorganic acids Inorganic alkali Inorganic oxides Inorganic salts Hot Products Lithium-bromide NEW.
It has a strong acidic nature and is corrosive. The most important carbon-carbon bond forming and breaking in biological chemistry encompasses gain or loss of a single carbon by an organic molecule in the form of CO 2.
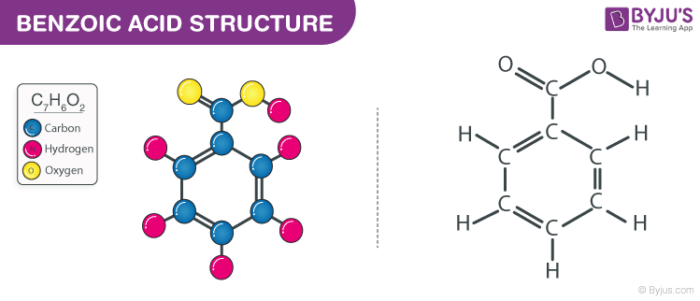
Benzoic Acid C6h5cooh Structure Properties And Uses Of Benzoic Acid

Benzoic Acid C6h5cooh Structure Properties And Uses Of Benzoic Acid
Benzoic Acid C7h6o2 Chemspider

0 Response to "Benzoic Acid Organic or Inorganic"
Post a Comment